We collaborated with Eli Lilly to present research on recombinant reagents and microfluidic technology for Bacterial Endotoxins Testing (BET) at the Parenteral Drug Association (PDA) Pharmaceutical Microbiology Conference.
Our findings, based on real-world sample data, demonstrate the accuracy and reliability of recombinant agents on modern microbiological detection platforms. Jay Bolden and Hayden Skalski present research at PDA Microbiology Conference.

Background: Limulus Amebocyte Lysate (LAL) vs. recombinant cascade reagents (rCR) in microfluidic BET assays
Pharmaceutical companies are increasingly adopting innovative technologies to meet evolving regulatory expectations while building more sustainable operations. Endotoxin testing is a prime example of this shift, as regulatory and sustainability pressures are transforming the BET landscape. Annex 1 encourages technological advancement to streamline manufacturing processes, and the recent publication of USP <86> on recombinant reagents for endotoxin testing represents a key opportunity to achieve both regulatory compliance and sustainability goals.
For these reasons, Veolia, in partnership with Eli Lilly, conducted a comparison study using the Sievers Eclipse Bacterial Endotoxins Testing (BET) Platform to compare results between traditional Limulus Amebocyte Lysate (LAL) and newer recombinant cascade reagents (rCR). The platform utilizes microfluidics and centripetal force; allows for assay set up in 85% of the time it takes to set up a traditional 96-well microplate; uses up to 90% less LAL or rCR; and automates the delivery of the LAL to samples. Beyond increasing efficiency, it assures precise and accurate results, allowing manufacturers to meet Annex 1 and sustainability goals while remaining in full compliance with regulations to assure patient safety.
This research outlines the results of the endotoxin tests with data obtained from real-world samples, providing a practical comparison between the two reagent types.
Comparison Study: Detecting naturally occurring endotoxins (NOE) and Reference Standard Endotoxin (RSE)
The purpose of this study was driven by two objectives. The primary goal was to evaluate the detection and recovery of both Naturally Occurring Endotoxins (NOE) and purified water spiked with Reference Standard Endotoxin (RSE). This evaluation was conducted using two different testing methods: traditional Limulus Amebocyte Lysate (LAL) and recombinant Cascade Reagent (rCR), with the goal of demonstrating reliable endotoxin recovery across both testing platforms.
Secondly, these capabilities were validated on the Sievers Eclipse BET platform by challenging it with both types of reagents. This validation was particularly significant as it aimed to demonstrate the system's suitability for real-world sample testing while offering two substantial benefits: a 90% reduction in reagent consumption and the option to use recombinant reagents, thereby promoting more sustainable testing practices in the industry.
Samples: rCR comparison study of 12 sample types
The following samples were used in the comparison study between Limulus Amebocyte Lysate (LAL) and recombinant Cascade Reagents (rCR):
- Two monoclonal antibodies
- Insulin
- Peptide
- NOE (see Figure 1)
- Histidine and Sodium Acetate
- LRW
- Purified Waters
- Purified Waters with RSE spikes
- Polysorbate 80
- Counterfeit products
- Components (stopper, cartridge)
- Yeastolate
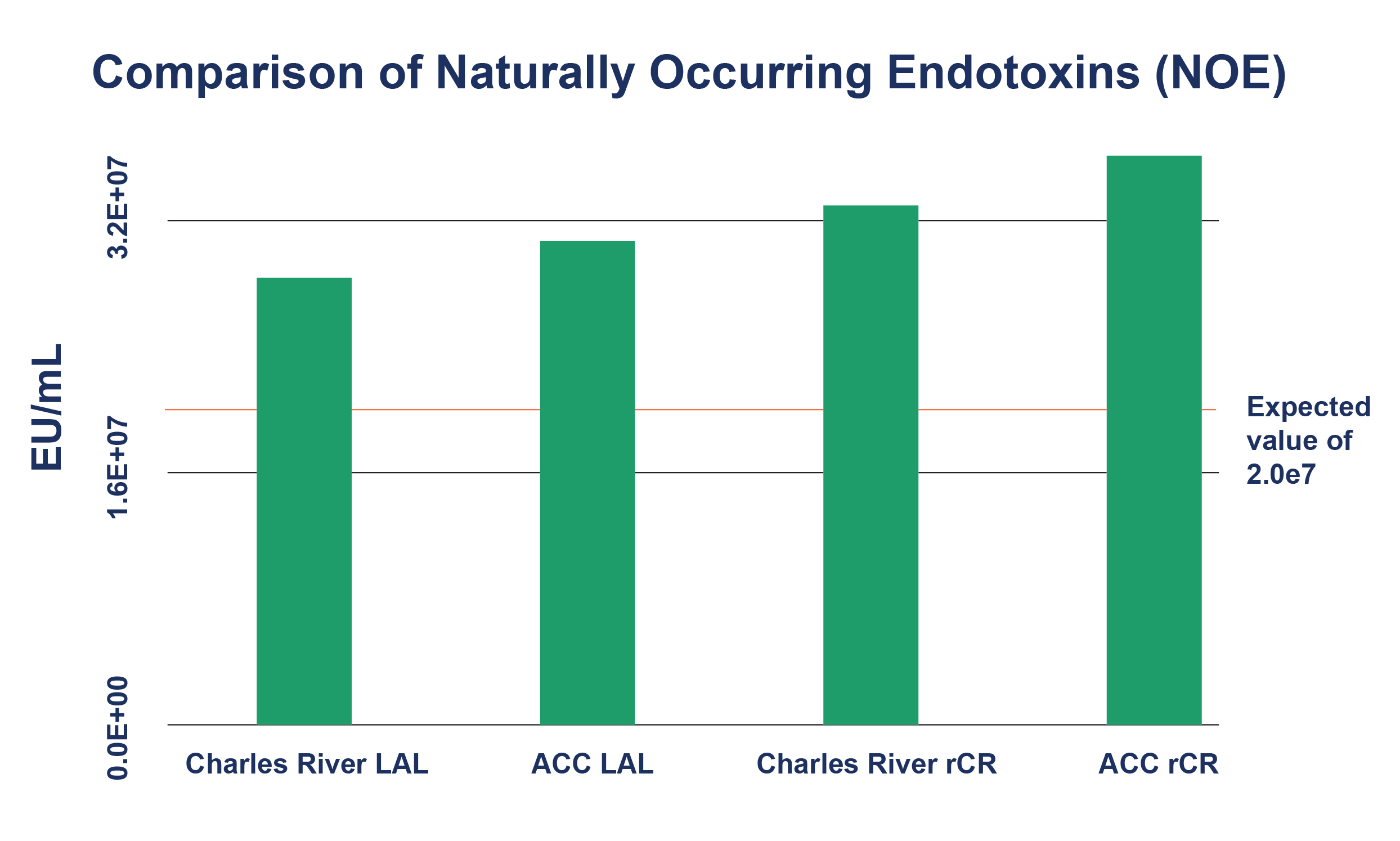
Figure 1: Comparison of Naturally Occurring Endotoxins (NOE)
Results: LAL vs rCR - Performance and testing results
The following figure details Positive Product Control (PPC) recovery rates of sample and reagents.
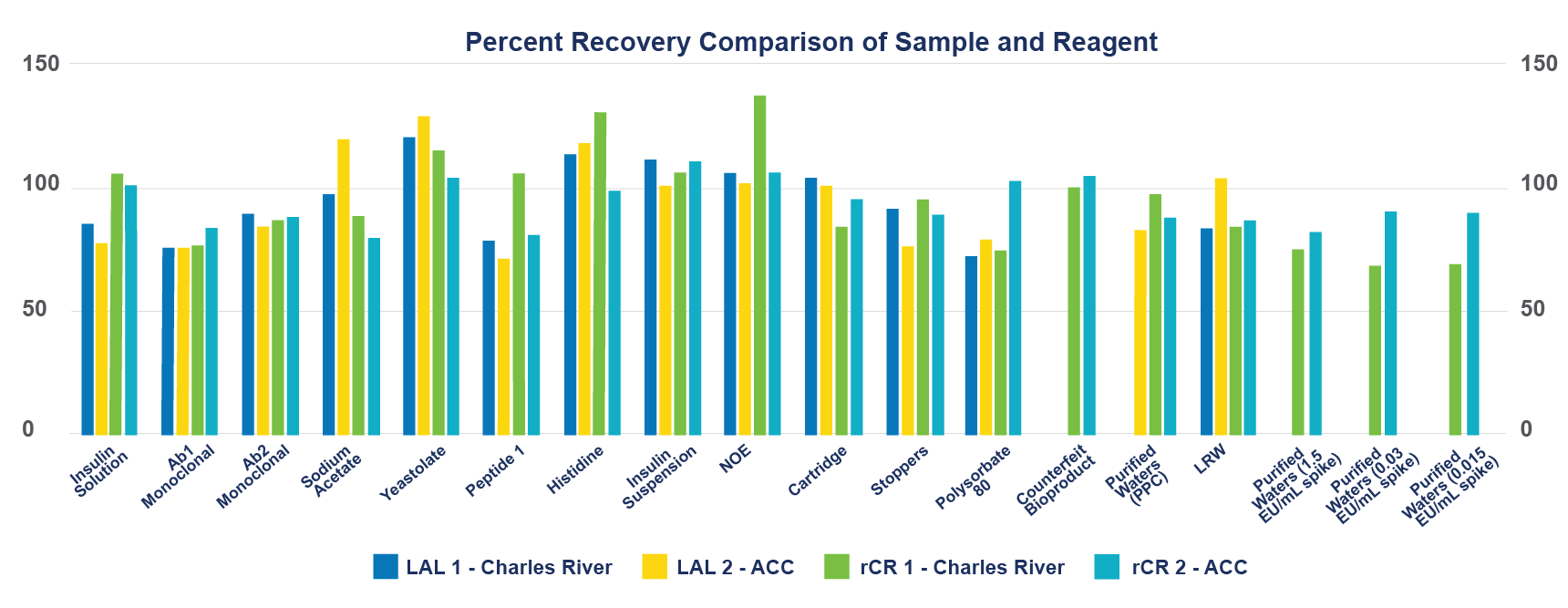
Conclusion: rCR performance in microfluidic platforms
This study showed equivalent performance between LAL and rCR using the Sievers Eclipse for the detection of bacterial endotoxins in real-world samples, as well as naturally occurring endotoxins. Based on the evidence, it can be determined that the Sievers Eclipse Bacterial Endotoxins Testing Platform is able to successfully utilize recombinant Cascade Reagents, provided that the sample's compatibility has been verified.
Automation via the Eclipse's centripetal microfluidic platform offers the simplest form of BET microfluidics available, providing significant time savings and reducing opportunities for error. With the availability of this innovative technology, BET assays can be streamlined while remaining fully compliant with compendia.
Os benefícios incluem:
- USP <85> and <86> compliant
- Proven to work with both LAL and rCR reagents
- Up to 90% less reagent needed; aids in sustainability initiatives
- 27 total pipetting steps for 21 samples for increased efficiency
- Innovative technology aligned with Annex 1 guidelines
Learn more about the Sievers Eclipse
Authors:
- Jay Bolden
-
Jay Bolden is a Senior Director in the Eli Lilly and Company global Analytical Quality Control Organization. He is a bacterial endotoxins subject matter expert and leads a team with global QC oversight for endotoxins, microbiology, and virology test methods. Jay holds a B.S. in Biology and an Environmental Studies certificate from Indiana University and has over 25 years of industry experience in development, process and laboratory microbiology, and microbiology laboratory leadership. Jay is a member of the United States Pharmacopoeia Microbiology Expert Committee and has authored a book chapter and multiple peer-reviewed articles on endotoxins. Conteúdo sanfonado 1.
- Meg Provenzano
-
Meg Provenzano is the Global Product Manager for Sievers bio-detection instruments at Veolia. Ela possui mais de 10 anos de experiência na indústria de testes de endotoxinas bacterianas e ocupou vários cargos em controle de qualidade, suporte técnico e gerenciamento de produtos. Prior to joining Veolia, Meg was a Product Manager with Charles River Laboratories. She is customer-centric and enjoys hands-on problem-solving, whether for technical issues, assay assistance, or software. Meg holds a B.S. in Marine Science and Biology from Coastal Carolina University, where she focused on Bottlenose Dolphin population research.
- Brian Short
-
Brian Short is a Global Pharmaceutical Application Specialist at Veolia, providing strategic pharmaceutical biodetection and TOC application support for the life science industry. With 20 years of experience working in and with pharmaceutical quality control laboratories, Brian has supervised and supported high-volume in-process and finished product testing. Having held previous roles with Wyeth and Lonza, and with nine years of experience supporting the Sievers product line as part of GE Analytical Instruments, SUEZ, and now Veolia, Brian has deep expertise in endotoxin detection instrumentation and software, including installation, qualification, and training. Brian holds a Bachelor of Science degree in Biological Sciences from York College of Pennsylvania.
- Hayden Skalski
-
Hayden Skalski is the Life Sciences Product Application Specialist for Veolia, specializing in bacterial endotoxins testing (BET). Hayden tem mais de 10 anos de experiência na indústria farmacêutica e em microbiologia de controle de qualidade e apresentou vários tópicos relacionados a testes de endotoxinas. Anteriormente, Hayden ocupou cargos na na Charles River Laboratories, na Regeneron e na Novartis, validando e executando protocolos de desenvolvimento de métodos para testes de endotoxinas, fornecendo suporte ao cliente, solução de problemas e suporte a testes de produtos de alto volume. Hayden é bacharel em Biologia pela Universidade de Albany (SUNY). Kelly Smith is a Senior Principal Biologist in the Eli Lilly and Company global Analytical Quality Control Organization. He is a bacterial endotoxins subject matter expert with over 25 years of industry experience and has a B.S. in chemistry from Butler University.
- Kelly Smith
-
Kelly Smith is a Senior Principal Biologist in the Eli Lilly and Company global Analytical Quality Control Organization. He is a bacterial endotoxins subject matter expert with over 25 years of industry experience and has a B.S. in chemistry from Butler University.
