The Fundamentals of Endotoxin Testing
What are endotoxins?
Endotoxins are biocontaminants derived from gram-negative bacteria's outer cell membrane. While not living organisms themselves, they are crucial for bacterial survival and serve multiple functions, including structural integrity and nutrient transport. Endotoxins are:
- Present naturally in food and water
- Dangerous if entering the bloodstream
- Common in raw materials and products
Endotoxins should not be confused with bacteria themselves - they are components that become harmful when separated from a bacterial cell.
Why do we test for endotoxins?
Bacterial Endotoxins Testing (BET) is crucial because endotoxins pose serious health risks when entering the bloodstream directly, bypassing normal digestive defenses. As pyrogens (substances that produce fever when introduced or released into the blood), they can cause potentially fatal drops in blood pressure, leading to organ failure, septic shock, or even death, when introduced into the bloodstream or spinal fluid.
Particularly concerning is that endotoxins persist even after sanitization; killing bacteria doesn't eliminate the toxic threat. For these reasons, testing is essential in these key areas of pharmaceutical and medical device manufacturing:
- Drug production and formulation
- Cleaning validation (equipment and container sanitization)
- Buffer preparation
- Drug reconstitution
Given endotoxins’ ubiquitous nature and resistance to standard sanitization methods, rigorous BET is vital for ensuring patient safety in medical and pharmaceutical applications.
Is endotoxin testing mandatory?
Regulatory agencies like the FDA, EMA, and others require endotoxin testing to ensure products meet safety standards and do not cause pyrogenic reactions. It is mandatory for:
- Pharmaceutical manufacturers producing parenteral drugs, including:
- Intravenous (IV)
- Intramuscular (IM)
- Intrathecal (IT)
- Medical device manufacturers whose products contact blood
- Veterinary/animal health product manufacturers producing blood-contacting items
The specific testing requirements depend on the product type, intended use, and regulatory jurisdiction.
How do you test for endotoxins?
Bacterial Endotoxins Testing (BET) primarily uses LAL (Limulus Amebocyte Lysate), derived from horseshoe crab blood. This remarkable discovery from the 1950s was standardized in the 1960s, creating a reliable endotoxins detection method.
There are three common testing approaches used today:
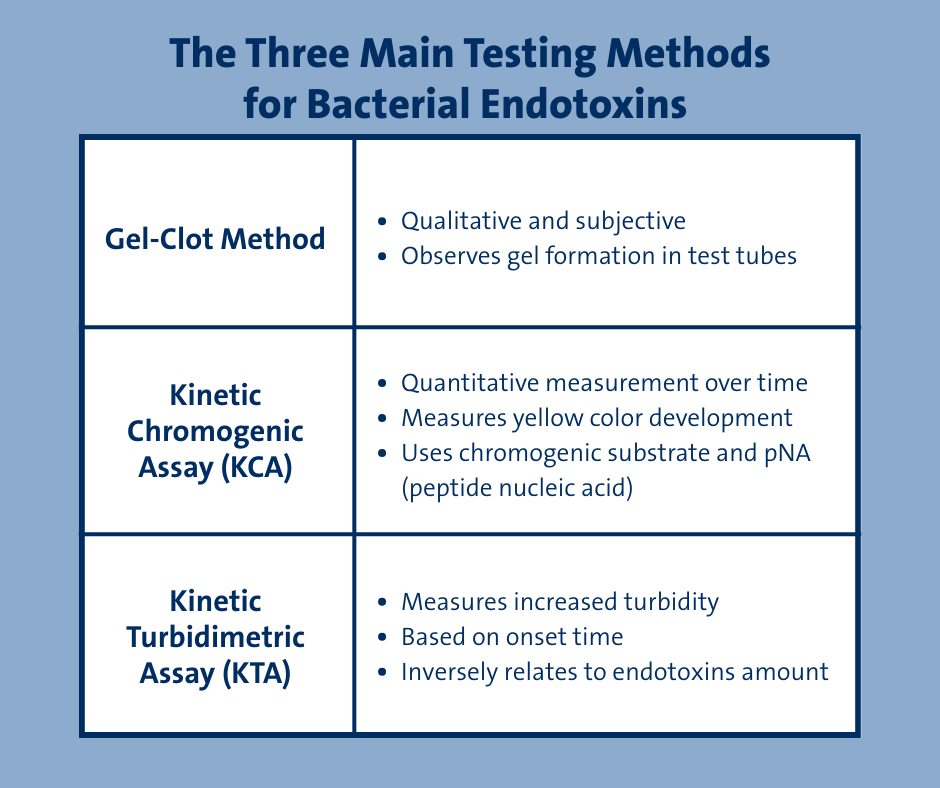
Deciding which method is best to use depends on several factors, including product characteristics, sensitivity requirements, and regulatory requirements.
The newest method for endotoxins testing: Microfluidic technology
The Sievers Eclipse Bacterial Endotoxins Testing (BET) Platform is a game-changing method for endotoxin testing due to its microfluidic technology that delivers significant time savings and efficiency improvements over traditional methods. Key advantages include an 89% reduction in pipetting steps and setup time of just 5-10 minutes. The Eclipse Platform uses a microfluidic plate to mix samples with LAL reagent, using only 1 mL of LAL reagent - 90% less than conventional methods - while maintaining accuracy. The unique microplate contains embedded endotoxin standards and positive product controls (PPCs) and remains stable at room temperature for up to 25 months, eliminating expensive cold storage requirements.
Other unique capabilities of the Eclipse Platform include remote data review, minimal laboratory space and training requirements, and reduced failure rates. Results are measured in Endotoxin Units (EU), with 1 EU approximately equal to 1 part per trillion (ppt). While LAL's biological nature typically requires users to prepare standard curves, PPCs, negative controls, and samples for each assay, the Sievers Eclipse significantly simplifies this process - users only need to add water and samples to the appropriate segments, as the standards and controls are already embedded in the microplate.

While traditional testing approaches work, newer microfluidic technology offers a more efficient, reliable, and cost-effective method for endotoxin testing while still ensuring regulatory compliance. Microfluidic technology also offers users flexibility in reagent choice, whether they choose traditional LAL or recombinant cascade reagent (rCR). This modern technology, combined with recombinant reagents, represents a key opportunity to achieve both efficiency gains and sustainability goals in endotoxin testing.
Learn more about bacterial endotoxins testing, LAL, rCR and the Sievers Eclipse BET Platform by watching a quick on-demand webinar: https://www.watertechnologies.com/lp-ai-usp-86-webinar
